|
The Basics
If the water is fit to drink it is probably suitable for brewing. But if you are trying to duplicate certain styles you will have make to some adjustments.
Chlorine
For all brewing it is best to remove as much chlorine as possible. Once in solution, chlorine may be present as chloride ions or hypochlorous acid. Chlorine will react with other compounds in the wort to form chlorophenols. If the chlorine in your water exists as free chlorine, you may boil the water. You can also let it sit in an uncovered container for at least 24 hours or you may use a carbon filter. Unfortunately, a lot water treatment plants now use a process called chloramination in which ammonia and chlorine are added to the water to produce chloramines. Chloramines are longer lasting than chlorine and may react with components in your beer much like chlorophenols. If your water is chloraminated, you will need a carbon filter to remove the chloramines because boiling does not work well.
Hardness
Hardness is a measure of the cations (positively charged ions) dissolved in the water. These are usually calcium and magnesium. The anion (negatively charged ions) associated with these cations determines if the hardness is permanent or temporary. If the anion is a bicarbonate, the hardness is temporary and can be removed. Boil the water and allow it to cool. If the predominant anion in your water is sulfate, the hardness is permanent and cannot be removed by boiling. If the calcium level is less than 100 ppm and the magnesium level is less than 30 ppm, you are okay. If calcium and/or magnesium levels are high, you may want to consider diluting your water with deionized or distilled water.
Water pH
pH is a numerical measurement of acidity or alkalinity determined by the presence of hydrogen ions.
This scale runs from 0 to 14, with 0 the most acidic and 14 the most basic or alkaline. To optimize enzyme activity and reduce extraction of husk tannins, a pH of 5.2 to 5.4 is recommended.
Homebrewing Water Treatment
Boiling - Bring water to a boil. Allow to cool and decant water off sediment. Removes bicarbonates and free chlorine.
Charcoal filter - Slowly run water through a activated charcoal filter. Removes organic impurites and free chlorine.
Standing - Allow water to stand uncovered for at least 24 hours. Only removes free chlorine.
Adjustment - Add specific acids and salts to alter water composition. To adjust pH to optimize the mash or to mimic the water of some other geographical location to reproduce a particular style of beer. pH may be adjusted directly by adding acid. Phosphoric and lactic acids are most commonly used.
Common Water Profiles for Brewing Regions and Some Common Styles
| By Region | Ca++ | Mg++ | Na+ | HCO3- - | SO4- - | Cl - |
|---|
| Burton | 268 | 62 | 30 | 141 | 638 | 36 |
| London | 90 | 4 | 24 | 123 | 58 | 8 |
| Edinburgh | 140 | 36 | 92 | 270 | 231 | 60 |
| Dortmund | 225 | 40 | 60 | 180 | 120 | 60 |
| Dublin | 52 | 16 | 99 | 156 | 77 | 1 |
| Munich | 75 | 18 | 2 | 150 | 10 | 2 |
| Pilsen | 7 | 2 | 2 | 15 | 5 | 5 |
| Yorkshire | 105 | 17 | 23 | 153 | 66 | 30 |
| Vienna | 200 | 60 | 8 | 120 | 125 | 12 |
| |
| By Style | Ca++ | Mg++ | Na+ | HCO3- - | SO4- - | Cl - |
|---|
| Pale Ale | 110 | 18 | 17 | 60 | 350 | 50 |
| Mild Ale | 75 | 12 | 35 | 100 | 120 | 100 |
| Stout/Porter | 50 | 12 | 60 | 150 | 46 | 175 |
| Pale Lager | 22 | 5 | 24 | 55 | 5 | 15 |
| Dark Lager | 5 | 12 | 35 | 100 | 120 | 100 |
Common Salts For Homebrewing Water Adjustments
| | 1 gram per gallon adds
___________________
|
|---|
Salt
Name | Common
Name | cations
in ppm | anions
in ppm |
|---|
| calcium sulfale | gypsum | 55 Ca++ | 132 SO4- - |
| calcium carbanate | chalk | 105 Ca++ | 158 CO3- - |
| calcium chloride | | 71 Ca++ | 126 Cl - |
| magnesium sulfate | Epsom salts | 26 Mg++ | 103 SO4- - |
| non-iodized salt | non-iodized
table salt | 103 Na+ | 160 Cl - |
Ground Water Hardness as CaCO3
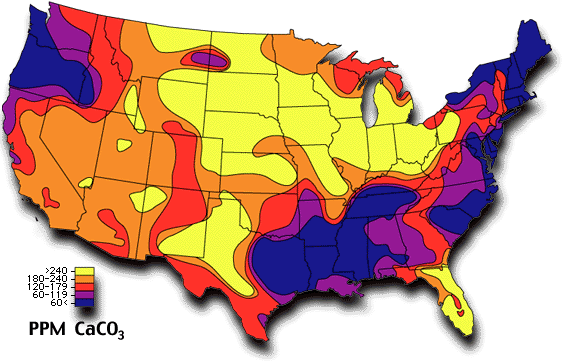
| Over 350 | Very Hard |
|---|
| 240-349 | Hard |
|---|
| 180-230 | Moderately Hard |
|---|
| 120-179 | Slightly Hard |
|---|
| 60-119 | Soft |
|---|
| Under 60 | Very Soft |
|---|
|
Water hardness is a common measurement of mineral levels in the water supply, expressed as parts-per-million CaCO3.
Temporary hardness is determined by the concentration of carbonate and bicarbonate. The hardness that carbonate and bicarbonate ions contribute is temporary because carbonate and bicarbonate are precipitated when water is boiled.
Permanent hardness is determined by the amount of calcium and magnesium ions present in the water.
|
|

